TEAR THROUGH RECOMMENDATIONS
Dermal Fillers for the Treatment of Tear Trough Deformity: A Review of Anatomy, Treatment Techniques, and their Outcomes
Abstract
Tear trough deformity is a major concern in a lot of individuals seeking periorbital rejuvenation. A prominent tear trough deformity is characterised by a sunken appearance of the eye that results in the casting of a dark shadow over the lower eyelid, giving the patient a fatigued appearance despite adequate rest, and is refractory to attempts at cosmetic concealment. The tear trough deformity is a natural consequence of the anatomic attachments of the periorbital tissues. A variety of techniques have evolved to address this cosmetic issue. Traditional techniques relied on surgical excision of skin, muscle, and fat as well as chemical peels. Treatment is now tailored towards specific anatomic abnormalities and often employs multiple modalities including surgery, botulinum toxin, and replacement of volume. Various original research articles, text book publications and review articles were studied. Data specific to the historical aspect and anatomy of tear trough have been enumerated. Techniques of different authors were analysed and their results and complications have been summarised. The technique of the author has also been described here.
INTRODUCTION
Tear trough deformity is a major concern in a lot of individuals seeking periorbital rejuvenation. A prominent tear trough deformity is characterised by a sunken appearance of the globe that results in the casting of a dark shadow over the lower eyelid, giving the patient a fatigued appearance despite adequate rest, and is refractory to attempts at cosmetic concealment. Under-eye circles caused by tear trough deformity can be confirmed and shown to the patient along with their disappearance when the patient is photographed using flash photography.[1]
The tear trough deformity is a natural consequence of the anatomic attachments of the periorbital tissues. A variety of techniques have evolved to address this cosmetic issue. Traditional techniques relied on surgical excision of skin, muscle, and fat as well as chemical peels. More recently, surgeons have better appreciated the need for restoration of volume of the orbit as part of an overall rejuvenation strategy. Treatment is now tailored towards specific anatomic abnormalities and often employs multiple modalities including surgery, botulinum toxin, and replacement of volume.
To quote Glaser et al., “The aesthetically attractive lower eyelid should display a relatively smooth transition between the pre-septal and orbital portions of the orbicularis oculi muscle and continue into the upper malar region without a definable transition point.”[2]
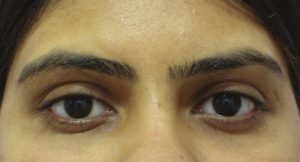
Dark circles are often a result of tear trough deformity [Figure 1]. However, the causes of dark circles can be multi-factorial. Changes in skin thickness, laxity, hyperpigmentation and actinic changes also play a role. Thin skin or prominent subcutaneous venous pooling accentuates the periorbital darkening.[1,3]
Tear trough deformity resulting in dark circles in a young Indian female
Additionally, prolapse of orbital fat may indirectly cause a shadowing over the lower lids.[4]
ANATOMY
Duke-Elder and Wybar first introduced the term nasojugal fold in 1961. They defined it as “running downwards and outwards from the inner canthus, the junction of the loose tissue of the lower lid with the denser structure of the cheek, marking the line along which the fascia is anchored to the periosteum between the muscles of the lid and those of the upper lip.”[5]
According to Loeb, the ‘nasojugal groove’ was caused by the following: (1) Fixation of the orbital septum at the level of the inferomedial portion of the arcus marginalis, (2) existence of a triangular gap limited by the lateral portion of the angular muscle on one side and the medial portion of the orbicularis oculi muscle on the other and (3) the absence of fat tissue from the central and medial fat pads subjacent to the orbicularis oculi muscle in the area below the groove.[6]
The term ‘tear trough deformity’ was coined by Flowers. In his opinion, descent of the cheek, loss of facial volume, underdevelopment of the infraorbital malar complex and a muscular defect between the orbicularis muscle and angular head of the quadratus labii superioris muscle were all responsible for the formation of tear trough deformity.[7]
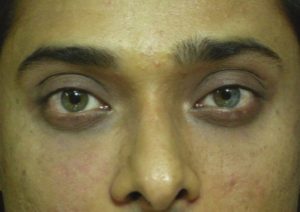
Haddock said the term ‘tear trough deformity’ should be applied to the medial periorbital hollow extending obliquely from the medial canthus to the midpupillary line [Figure 2]. Lateral to this, the depression is better referred to as the ‘palpebromalar groove’, ‘nasojugal groove’, or the ‘lid-cheek junction’.[8–11]

In their cadaveric dissection studies, Muzaffar et al. describe an orbicularis retaining ligament in the lower eyelid that originates laterally as a reflection of the septum orbitale joining a membrane derived from the preperiosteal fat over the zygoma.[10] The medial extent of this retaining ligament is variable, connecting with the orbicularis oculi muscle insertion and thus indirectly to the medial canthus. The central portion of the retaining ligament is the weakest and distends differentially more with age, allowing for greater exposure of the central fat pad. Greater laxity of the lid-cheek junction with age contributes to the tear trough deformity by accentuating the herniation of orbital fat.[10] Midface ptosis helps to bare the tear trough, as involutional descent of the midface with the orbicularis muscle tethered over the tear trough by the orbicularis retaining ligament results in thinning of the tissues over the tear trough and increased prominence, readily recognizable as a sign of ageing.[12]
Kane describes the tear trough as a depression centred over the medial inferior orbital rim and bounded superiorly by the infraorbital fat protuberance. As the patient ages, the globe descends within the orbit and the infraorbital fat is displaced anteriorly. This anterior bulge causes the tear trough to deepen. Because this protuberance creates shadowing below it, it causes the apparent tear trough deformity to deepen further, depending on the conditions of lighting. The inferior border of the tear trough is formed by the thick skin of the upper cheek with its abundant subcutaneous fat, suborbicularis oculi fat and portions of the malar fat pad. In most individuals, the trough is deeper medially, becoming shallower laterally. The thin skin at the depth of this groove has very little fat beneath it, contributing to the apparent depression. With age, further loss of soft tissue and, importantly, a loss of osseous support also causes the tear trough to deepen further.[13]
Sadick et al. proposed a tear trough rating scale (TTRS). According to this TTRS, it was found that the tear trough was not simply an age-related deformity. It is often related to a forward projection of the bone of the upper cheek, and it may be more common in patients with either congenital or age-related maxillary hypoplasia. They said that the tear trough should be defined as the depression of the medial lower eyelid just lateral to the anterior lacrimal crest and limited in its inferior aspect by the inferior orbital rim. This region corresponds anatomically to where the lacrimal sac lies; hence the term tear trough.[1] In a recent publication, Wong et al. described a tear trough ligament after dissecting 48 cadaveric hemi faces. A true osteocutaneous ligament called the tear trough ligament was consistently found on the maxilla, between the palpebral and orbital parts of the orbicularis oculi, cephalad and caudal to the ligament, respectively. It commences medially, at the level of the insertion of the medial canthal tendon, just inferior to the anterior lacrimal crest, to approximately the medial-pupil line, where it continues laterally as the bilayered orbicularis retaining ligament. Histologic evaluation confirmed the ligamentous nature of the tear trough ligament, with features identical to those of the zygomatic ligament.[14]
CLASSIFICATION
In an effort to objectively analyse their postoperative results, Barton et al. proposed a grading system based on anatomic analysis [Table 1].[15]
Table 1
Barton’s grading system based on anatomic analysis

Sadick et al. developed the TTRS by objectively and subjectively evaluating the clinical appearance of the tear trough with regard to depth of the trough, hyperpigmentation, volume of prolapsed fat and skin rhytidosis.[1]
A numerical score was then assigned with respect to severity, depth of the tear trough, distance from the anterior lacrimal crest to the depth of the trough; each millimetre of depth is given one point.
Hyperpigmentation
Dyspigmentation, although not directly contributing to the depth of the trough, creates an illusion of depth; no hyperpigmentation is given one point, mild is given two points, moderate hyperpigmentation is given three points and intense or deep hyperpigmentation is given four points; subdermal dark casting caused by venous pooling can also be graded as hyperpigmentation.
Prolapse of nasal fat pad/pockets
Prominent prolapse of the nasal fat pad accentuates the depth of the trough and is rated as mild (one point), moderate (two points) or severe (three points).
Rhytidosis
Lower eyelid skin rhytidosis accentuates the fatty prolapse and the depth of the trough; skin rhytidosis is rated on a scale of one to four (mild, moderate, advanced and severe, according to Glogau scale) and the rating corresponds to the number of points assigned.
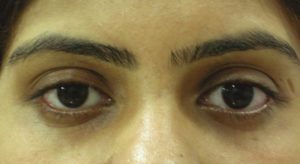
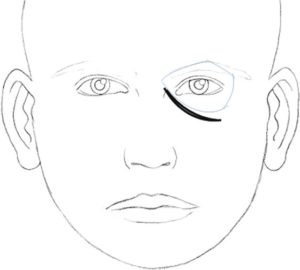
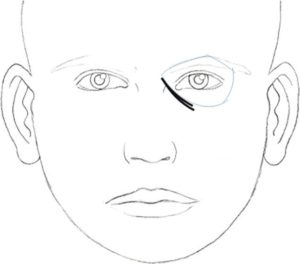
In 2010, Hirmand proposed a classification system of the tear trough deformity based on clinical evaluation[16] [Figures [Figures33–5, Table 2].
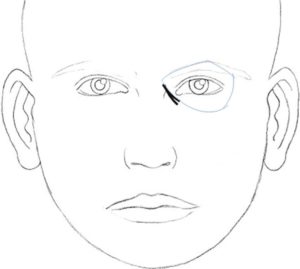
Hirmand’s class 1
Hirmand’s class 3
Table 2
Hirmand’s classification system of the tear trough deformity based on clinical evaluation


Hirmand’s class 2
CONTRAINDICATIONS
-
Unrealistic expectations
-
Infection near the site of injection
-
Known allergy or hypersensitivity to the material or to the lidocaine mixed in the syringe of the filler[17]
-
Patients with septal fat herniation
-
Severe elastosis (e.g., dermatochalasis or large eye bags)[18]
PRECAUTIONS
-
Vitamin E, gingko biloba, aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided at least five days before the treatment to prevent bruising.
-
Care should be taken in patients with a history of lower eyelid blepharoplasty without lateral retinacular suspension.
-
One should be cautious while injecting around the infraorbital foramen to avoid injury to the neurovascular bundle.
-
Careful and gentle moulding of the implant for a more homogeneous distribution of the material is encouraged after injection to obtain an even distribution of the filler in the lateral part of the hollow.
TECHNIQUES
In a 2006 multispecialty consensus recommendation article, the tear trough was listed as the most challenging area to treat with hyaluronic acid (HA).[19] The currently popular HA fillers are produced by bacterial fermentation (Streptococcus strains) and stabilised by chemical cross-linking. The nonanimal-derived products have substantially decreased allergic reactions.[20] The products differ in their cross-linking methods, concentrations, and size of particles.[21,22]
Lambros’s technique
Lambros stressed that when one engages in nonsurgical treatment for tear trough correction, it is important to evaluate the following factors:[23]
-
Skin quality, as patients with thick, smooth skin will have better results than those with thin extremely wrinkled skin.
-
Definition of the hollow, as a more defined hollow is more amenable to fillers.
-
The orbital fat pad, as larger fat pads are more difficult to correct due to ‘puffiness’ caused by the injection.
-
The colour of the overlying skin, as the filler may improve shadowing but will not improve dark pigmentation.
After application of an ice pack to the lower lid and cheek, a local anesthetic consisting of 0.5% lidocaine with epinephrine (0.2 to 0.4 mL) is injected into the orbicularis within the boundaries of the tear trough. Finger pressure is applied to flatten the area of injection. A half-inch, 30-gauge needle is inserted through the skin at the most lateral extent of the tear trough, advancing fully and potentially indenting the skin with the hub for full reach. The HA is then injected deep into the dermis as the needle is withdrawn. This process is repeated above and below the original site of the injection. The area is then inspected, and additional passes are made as needed to yield a smooth contour. Last, the area is massaged lightly, compressed with finger pressure, and rolled with a cotton applicator. In his description of the technique, Lambros stressed the importance of not forcefully compressing the product during massage, as this can displace the product into the cheek and exaggerate the tear trough. Postinjection care involves applying ice to the area the night of the procedure, and patients are instructed to refrain from massaging the area.
Kane’s technique
After evaluation and marking of the tear trough, betacaine topical anaesthetic ointment is applied to the lower eyelids at least 20 minutes before the injection.[13] After preparation of skin with alcohol, a 30- or 32-gauge needle is inserted for injection. The skin of the lower lid is spread and held at some tension with the noninjecting hand. The skin is inspected carefully for visible vessels before each needle stick. The deepest portion of the medial tear trough is treated first. The needle is threaded below the surface of the skin above the orbicularis oculi. A miniscule amount of hyaluronic filler is injected at each pass. Parallel threads of the filler are injected cephalad and caudal to the tear trough. The raised area of the filler is then tapered off medially along the nasal sidewall superiorly at the most cephalad-significant rhytid, inferiorly at least abutting or immediately caudal to the thick skin of the cheek, and laterally to at least the junction of the medial and lateral third of the inferior orbital rim. If the tear trough is deep, the direction of the needle is changed throughout the injection so that the filler is applied in a cross-hatched fashion. The volume range is 0.1 to 0.45 mL per eyelid, with most patients requiring 0.2 to 0.3 mL.
Technique of stutman and codner
With the patient seated, the tear trough deformity and lid-cheek junction are marked with easily removable white eyeliner. The patient is advised to apply ice packs to the area several minutes before injection to minimise bruising and for anaesthetic purposes. After the markings are confirmed by the patient, HA is injected deep in the preperiosteal plane, to reduce visibility of the product. The HA is placed beneath the insertion of the medial orbicularis muscle at the maxilla and continues laterally inferior to the orbicularis retaining ligament. A combination of crosshatching and linear threading is utilised with a 30-gauge needle with care not to inject superficially. The product is lightly massaged with cotton-tipped applicators to disperse any visible irregularities. After injection, the patient is instructed to apply ice to the area over the next 24 hours as needed to decrease oedema and ecchymosis.[24]
The technique of kenneth and samantha steinsapir
Kenneth and Samantha reported a technique of deep HA filler injections in 164 patients with tear trough. The mean dose of filler per session was 1.53, 0.8, 0.84 and 0.38 mL divided between the two lower eyelids. The goal was to place aliquots of the filler in the preperiosteal tissues just inferior to the orbital rim. It was sometimes necessary to digitally elevate the inferior orbital fat in the lower eyelid to expose the desired injection site. The bony orbital rim is free of significant vascular structures from the base of the anterior lacrimal crest to the lateral canthal tendon. The filler was introduced by using a serial puncture technique. Patients were permitted to close their eyes. The orbital rim was digitally palpated and the needle rotated so that the bevel was parallel to the skin and advanced to be flush on the periosteum. Before injection, the impaled soft tissue was digitally pulled over the needle like curtains over a curtain rod (curtain manoeuvre). This decreased the risk of backflow of the filler in a more superficial plane. At each site, approximately 0.1 mL was injected. The needle was withdrawn and the filler moulded to the desired contour.[25]
Technique of patel and glaser
Patel and Glaser have described a similar technique. HA filler was injected by using a serial point injection or linear threading technique and massaged using a cotton-tipped swab or a digit. Small aliquots of filler just inferior to the orbital rim at a plane between the periosteum and the orbicularis oculi muscle were also injected to correct loss of volume in the lower eyelid area due to pseudoherniation, orbital septal laxity and atrophy of the midface. The filler material was carefully massaged to allow even distribution.[2]
Consensus group technique: Anatomic guidelines for augmentation of the cheek and infraorbital hollow
To be able to teach others how to improve aesthetic outcomes in the midface and infraorbital hollows, a consensus group of European and North and South American aesthetic experts convened at an academic workshop to develop keys to optimal outcomes. The best practice guidelines for midface and infraorbital hollow injections were discussed. The consensus group recommend vertical supraperiosteal depot technique (VSDT) or linear threading for infraorbital hollow augmentation.
VSDT uses only single, small depots of soft tissue filler material that are placed via a vertical injection on a location directly on the bone or, more exactly, on the periosteum. Due to the bony support, only very little material is needed to produce a pronounced correction at the surface of the skin. The intention in using this technique is to avoid overcorrection.[26]
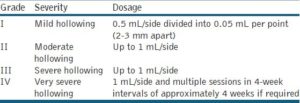
According to the consensus team, the filler should be injected at the supraperiosteal level along or below the orbital rim under the defect or both, protecting the edge of the rim to prevent deposition of filler above that structure.[27] Most of the filler should be injected underneath the orbicularis oculi muscle. However, the medial part of the muscle, which attaches to the bone, requires direct injection into the muscle itself. Serial injections using VSDT, implanting 0.02 to 0.05 mL per perpendicular injection point just above the bone 2 to 3 mm apart, are recommended. The linear technique is preferred if the cheek has been augmented well, using an entry point beneath the lateral and, in some cases, also the medial canthus. The cannula (or needle) should be placed perpendicular to the skin, advanced to the periosteum, and moved forward until it reaches the top of the nasojugal fold. This technique allows for deep injection using retrograde linear threading along the orbital rim, as described above. The injected volume depends on the severity of hollowing [Table 3]. Repeated injections may be required for optimal augmentation.
Patrick Trevidic has reported the use of blunt-tipped cannulas for tear trough correction with HA fillers.[28] Blunt-tipped cannulas are safer, because the chance of injuring vessels and nerves is smaller than with needles. It is less painful and produces less oedema.
The author’s technique
Patients on vitamin E, gingko biloba and NSAIDs are asked to discontinue the same for a week before the treatment to prevent bruising.
All the patients are photographed with a standard powershot camera with the same settings and adequate lighting. Written informed consent is taken.
The author prefers to inject with the patient reclined at 45° as the tear trough deformity is better visible in this position than when the patient is lying down. Good lighting is extremely important for better visibility of anatomical landmarks. The injections should not be given in a hurry as chances of hitting blood vessels or injecting superficially are higher when in haste.
Any cosmetic makeup in the area to be treated is completely removed with a cleansing lotion. Cleansing is further done with chlorhexidine and normal saline. The area to be treated is numbed with ice cubes. The orbital rim is palpated. Due to the rich subdermal vascular plexus, the tear trough area is prone to significant bruising. Hence, the author prefers to keep the point of insertion of the needle about 1.5 cm below the orbital rim in line with the midpupillary line. This reduces the chances of bruising [Figures [Figures66 and and77].
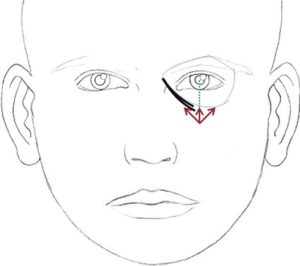
Marking of tear trough filler (injection points and direction of needle)
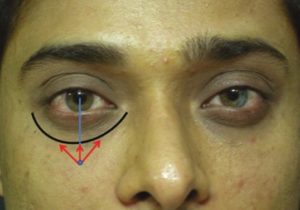
Marking of tear trough filler
Cross-linked HA with lignocaine (Juvederm Ultra@ XC) is injected with a 30-gauge needle. Juvederm@ Ultra XC has a cross-linking process called Hylacross™ which provides a concentration of 24 mg/mL of HA. The 6% cross-linked composition produces a soft, viscous, nonbeaded gel which helps in smooth injection and is intended to enhance durability. It also has 0.3% preservative free lidocaine which helps in alleviating pain during and after the injection. The patient is comfortable during the procedure. Patient compliance improves as well.
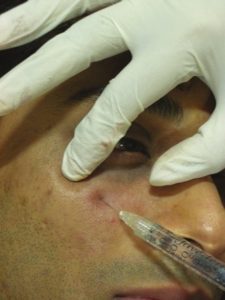
The needle is directed diagonally up towards the medial canthus and plunged deep into the skin through the muscle right up to the periosteum. HA is deposited subperiosteally to a visual end point of optimal correction. Protection of the globe is ensured by palpating the infraorbital rim with the noninjecting hand [Figure 8].
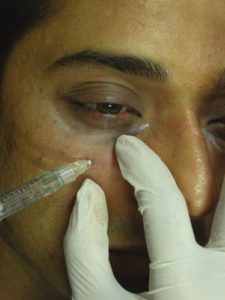
Subperiosteal injection of hyaluronic acid filler towards medial canthus, note the patient is comfortable even while injecting due to premixed lidocaine present in the syringe
The needle is slowly withdrawn and the material is not injected while the needle is being withdrawn, as superficial injections are bound to give a Tyndall effect in this area. The direction of the needle is then changed vertically up towards the midpupillary line and plunged again into the periosteum. Another depot injection is given [Figure 9]. A third injection is given only if there is loss of tissue below the lateral orbital rim. The direction of the needle is changed diagonally up towards the lateral canthus to give another depot subperiosteally [Figure 10]. Usually about 0.2 mL is adequate per depot. The area is gently massaged for an even distribution of the product [Figure 11]. This also ensures that there are no lumps or irregularities. However, vigorous massage should be avoided in this area as it can push the substance onto the globe. Care is taken to inject away from any visible blood vessels in this area. Ice packs can be applied to the treated area to reduce oedema, bruising and discomfort. The patients are asked to avoid massage or any facial treatments for a week.
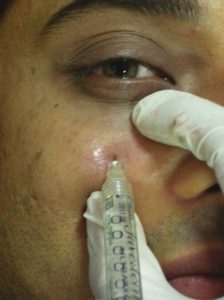
Subperiosteal injection of hyaluronic acid filler towards midpupillary line
Subperiosteal injection of hyaluronic acid filler towards lateral canthus
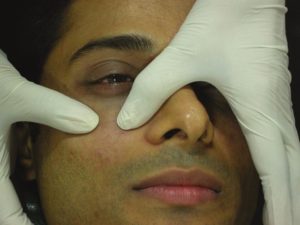
Gentle massage of the filler for even distribution
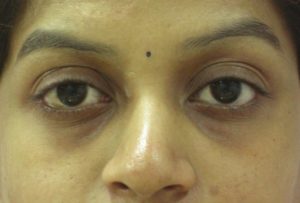
The patient is asked to follow up in 15 days to see if a touch-up is required. HA is injected until the point of full correction, if necessary. The patients are asked to follow up again in 6, 12, 15 and 18 months. The observation of the author has been that a HA in the tear trough area usually lasts for up to 12-15 months [Figures [Figures1212–15].
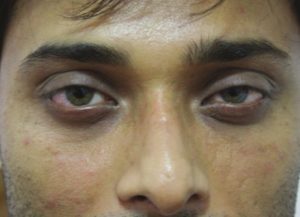
Male: Before tear trough filler treatment