AUTOLOGOUS FAT TRANSFER DATA
The science behind autologous fat grafting
Referred to by
Peer review report 3 on “The Science Behind Autologous Fat Grafting”
Peer review report 2 on “The Science Behind Autologous Fat Grafting”
Peer review report 1 on “The Science Behind Autologous Fat Grafting”
Highlights
- •
-
This review analyse the historical evolution of the surgical harvesting and implant technique.
- •
-
It focuses on the technical improvement to obtain a better fat grafting survival.
- •
-
Adipose derived stem cells and their regenerative properties are also reported.
Abstract
Introduction
Adipose grafting has undergone significant changes over time. Many different techniques have been followed by trying to improve the quality of the lipoaspirate and the survival of the fat graft after implantation.
Material and methods
The purpose of this review is to analyse the historical evolution of the surgical harvesting and implant technique, describing the changes that have brought significant improvements, revolutionizing the aesthetic and functional results obtainable.
Results
A standard fat grafting technique is commonly performed in three stages: harvesting of adipose tissue from a suitable donor site; processing of the lipoaspirate to eliminate cellular debris, acellular oil and excess of infiltrated solution, reinjection of the purified adipose tissue. The most widely used surgical technique was described by Coleman. He modified and corrected the methods and results of his predecessors and proposed an atraumatic protocol for the treatment of adipose tissue.
He reported that the key to successful fat grafting lies in the technique. In addition, he noticed that adipose tissue was not only a good filler, but improved the quality of the skin. In fact, fat grafts demonstrated to have not only dermal filler properties but also regenerative potential owing to the presence of stem cells in fat tissue.
Conclusion
Adipose tissue, actually, is the closest to the ideal filler because it is readily available; easily obtainable, with low donor-site morbidity; repeatable; inexpensive; versatile; and biocompatible. There is an abundance of literature supporting the efficacy of fat grafting in both aesthetic and reconstructive cases. Recent studies have shown the utility of adipose-derived stem cells in the improvement of wound healing, describing their ability to regenerate soft tissues and their remodelling capacity provided by their unique cytokine and growth factor profiles.
Despite ongoing concerns about survival and longevity of fat grafts after implantation and unpredictability of long-term outcome, fat has been successfully used as a filler in many differ clinic situation.
Keywords
1. Introduction
Subcutaneous adipose tissue is a soft and malleable tissue, and it is generally present in the body in large quantities making it the ideal filler for correcting and remodelling profile and volume body defects.
The first attempts to transfer adipose tissue date back to the end of the twentieth century [1]. In 1889, Van der Meulen [1], [2] first attempted to a fat auto-transplantation. He performed a free omentum and autologous fat grafting between the liver and the diaphragm to treat a diaphragmatic hernia.
Neuber [3] made the first true adipose graft in 1893. He took small fat grafts from the forearm and used them to fill a depressed scar on the face, resulting in tuberous osteitis. He was the first to note that using large grafts the result was unsuccessful, restricting to use small and sufficient graft he obtained excellent aesthetic results [1].
During the first half of the 20th century fat transplantation became popular among many medical specialties. These procedures involved en bloc transplantation of fat harvested through an incision in the donor region. Transplantation to cutaneous and subcutaneous defects also involved an incision in the recipient site through which to implant the fat with a variable fat transplanted survival rate.
In 1910, Lexer [4] published an article describing for the first time the use of adipose tissue in aesthetic surgery to correct aging defects. I used fat as a filler for the malar infraorbital area, to stretch the grooves and wrinkles of the face. He harvested 12 × 12 cm autogenous fat graft from abdomen. After that, he published a study on the survival of adipose grafts, demonstrating that the tissue should not be damaged during picking or during planting to obtain a good response [5], [6].
Brunning [7] introduced in 1911 the use of a syringe as an instrument for the fat grafting; he was the first to inject autologous fat into subcutaneous space. He used small fragments of adipose tissue to correct the aesthetic outcomes of rhinoplasty. He noted, however, that the good results obtained were lost with the reabsorption of grafting [6].
In 1912, Eugene Holländer (1867–1932) from Berlin published photographic documentation of natural appearing changes after infiltration of fat into two patients with lipoatrophy of the face [8]. In 1926, Charles Conrad Miller [9] wrote about his experiences with infiltration of fatty tissue through cannulas in the correction of scar contraction on the face and neck.
The use of autologous adipose grafts is also reported during World War II, when Salvat made camouflage allied spies injecting their fatty tissue into the face. He used autologous fat tissue for permanent results and homologous fat for temporary effects [6].
In 1895, Czerny [10] reported the first case of breast reconstruction with autologous fat tissue. He used a massive lipoma removed from the back, to fill the loss of substance as a result of breast tumorectomy.
Lexer, in 1931 [11], presented a case of chronic cystic mastitis that was completely reconstructed by adipose tissue. He removed all the glandular material of the breast and then rotated fat from axilla into the defect [1].
In 1941, May described the use of a large free fat graft on one side and a free fascia-fat autograft on the other from the posterolateral surface of the thigh for a bilateral breast reconstruction. He believed that fascia could preserve adipose tissue, reducing its absorption [1].
In the first half of the twentieth century, many authors including Eitner [12], Peer [13], Boering, Huffstadt [14], and Sawhney [15], experienced the use of dermofat grafts obtaining good results [6]. Bames [16], in 1953, and Scorocher [17], in 1957, reported few cases of fat grafting for breast augmentation and, also, Peer [13] experienced a dermofat grafting for the treatment of Poland’s syndrome.
In 1975, Arpad and Giorgio Fischer [18], father and son cosmetic surgeons, developed the modern technique of liposuction. They was the first to introduce blunt hollow cannula attached to a suction source and the criss-cross suctioning technique from multiple incision sites.
Illouz [19] modified and popularized the Fischer’s technique and in 1977, he developed modified equipment for performing liposuction making the technique less traumatic and reducing hemorrhagic risk. He was the first to use liposuction tissue as a filling product; with this technique, fat could now be transplanted without donor or recipient incisions. The Brazilian Luiz Toledo, in 1988 [20], experienced the use of disposable syringes of different gauges and size for aspiration of adipose tissue. The main advantage of syringe liposuction is, therefore, the precision and accuracy in measurement of adipose harvested volumes, in addition to the possibility of injecting fat with a less traumatic procedure [21].
A year later, Fournier [22], [23], [24] proposed a new technique for infiltrating adipose tissue, calling it lipofilling. The need for hypercorrection and the enormous variability of results did not allow its broader development in those years.
Bircoll [25] was the first to associate liposuction technique with autologous adipose graft in the breast augmentation, for the treatment of breast hypoplasia or symmetrisation after malignancy surgery. He proposed injections of small amounts of adipose tissue in different sessions.
Other authors after him experimented with the application of autologous and heterologous grafts in the breast [26], [27], [28].
Ellenbogen [29], in 1986, reported the successfully use of free autogenous fat grafts between 4 and 6 mm (the size of a pearl) to correct pitting acne, nasolabial folds, eyeliddepressions, facial atrophy, facial wrinkles, depressed scars, and in chin augmentation.
Peer [13], [30] studied fat transplantation extensively in the middle of the 20th century and hypothesized a 50% weight and volume loss of the fat graft after 1 year. Frustration with variable survival and the cosmetic problems of both donor and recipient incisions lead to physician disappointment with fat grafting by the 1950s.
The radical change in the history of fat transplantation depends on the publication of Coleman’s studies [31], [32], [33], [34]. Since 1986 he modified and corrected the methods and results of his predecessors and proposed a traumatic protocol for the treatment of adipose tissue.
He reported that the key to successful fat grafting lies in the technique. Harvesting, refinement, and transfer of subcutaneous tissue to provide pure, intact parcels of fat are essential for successful fat grafting. The surgeon also must infiltrate the refined fat parcels into the recipient site so that they survive predictably and uniformly, become integrated into the host tissues, and accomplish the desired structural alteration. The key to attaining these goals is the placement of minuscule amounts of fatty tissue with each withdrawal of the infiltrating cannula [31], [32], [33], [34].
2. Fat survival
In clinical practice, the main problem emerging after adipose tissue autotransplantation is its absorption rate over time: an average reduction observed varies from 25 to 70% of the total implanted volume [13], [30], [35].
In 1987, the American Society of Plastic and Reconstructive Surgeons reported the problems and difficulties that have been encountered with autologous fat transplantation and concludes that only 30% of injected fat can be expected to survive for 1-year [36].
Two different theories on adipocytes survival after fat transplantation have been advanced.
In 1923, Neuhof and Hirshfeld [37] examined grafted fat under a microscope and observed that in the first months after transplantation the tissue was dominated by degenerative phenomena with evident cellular suffering. After a few months began to be evident the first regenerative phenomena. Degenerative cells stimulate the inflow of histiocytes to phagocytize cellular debris and they noticed that some wandering cells underwent characteristic changes into embryonal fat cells which eventually became adult fat tissue [38].
They observed complete regeneration of the adipose tissue after 5 months and the metaplastic fat assumed the characteristics of the normal mature adipose tissue.
They also observed that occasionally, instead of becoming normal-appearing fat, the grafted tissue became permeated with connective tissue. They concluded that transplanted fat completely died and was replaced by fibrous tissue or newly formed metaplastic fat. This came to be known as the “host cell replacement theory” of fat grafting.
Cellular survival theory introduced by Peer, on the contrary, argues that the final volume that can be obtained after an adipose tissue transplant depends on the number of vital adipocytes present at the time of transplantation [39].
On the first day, grafted adipocytes pass through an ischemic phase. There is a recall of macrophages, histiocytes, and polynucleated cells for phagocytosis. On the fourth day, revascularization of the graft is done by neoangiogenesis of the host. This mechanism takes place centripetally and starts from the periphery. Central adipose tissue undergoes a longer period of ischemia and vascularizes only when the fragments are small [39].
Studies on cell viability have shown that mature adipocytes are very fragile cells and have a low level of resistance to trauma and ischemia [40], [41], [42].
Preadipocytes are more resistant to hypoxic and traumatic insults caused by the procedures for taking, processing and replanting: this is because all progenitor immature cells have a minimal metabolic activity they are able to survive without nutrition much longer and have a much lower oxygen consumption rate than mature adipocytes [40], [41], [42].
Preadipocytes and adipose-derived stem cells might be the only tissue that survives transplantation, and the variability of these cells between individuals may be one of the reasons for the observed variability of survival of fat grafts. Because adipose-derived stem cells are so much more tenacious than lipid filled adult adipocytes, some researchers believe that a major effect of fat tissue transplantation is caused by the survival of adipose-derived stem cells in the stromal cell fraction [38].
Current literature describes a newly-placed fat graft as consisting of three zones: an outer, “surviving” zone, an intermediate, “regenerating” zone, and a central, necrotic zone. According to Eto et al., the overall volume of a fat graft retained depends on the degree of survival of the regenerating zone, which contains adipose derived stromal cells (ASCs) with the potential for differentiation and replacement of adipocytes lost in the necrotic zone [43].
All graft adipocytes died and were replaced by differentiation of ASCs within the regenerating zone [44]. In addition to contributing to adipogenesis within transplanted adipose tissue, ASCs have also been implicated in encouraging graft revascularization via paracrine effects [45], [46], [47]. Recent experimental studies [48], [49] demonstrate the fundamental role that the vascular stromal fraction has in the long-term survival of the transplanted fat tissue. This, in fact, is an indispensable factor in inducing structural remodelling and cellular morphogenesis in the host tissue and in its absence, no satisfactory results are obtained in the fat survival [40].
The Stromal Vascular Fraction (SVF) therefore has an important regenerative function that also exerts paracrine secretion of several factors such as VEGF, HGF and TGF-β, which are released following various stimuli, including hypoxia, and that strongly affect the differentiation of stem cells, induce angiogenesis, stimulate tissue remodelling and heal wounds [50], [51], [52], [53], [54], [55], [56], [57].
3. Adipose derived stem cells
Recent studies reported that fatty tissue has the highest percentage of adult stem cells of any tissue in the body, with as many as 5000 adipose-derived stem cells per gram of fat compared with 100–1000 stem cells per millilitre of bone marrow [58], [38].
ASCs are similar to bone marrow derived stem cells in that they are capable of differentiating into multiple mesodermal tissue types and show similar surface protein marker expression [53].
Many studies demonstrated the ability of adipose-derived stem cells to undergo multilineage differentiation, not just into fat but also into bone, cartilage, skeletal muscle, cardiac muscle, blood vessels, nerves and skin [54], [55], [56], [57], [58], [59]. Furthermore, adult, lipid-filled adipocytes can dedifferentiate into stem cells and redifferentiate into other tissues such as bone [60].
ASCs are different from bone marrow derived mesenchymal stem cells because they can be easily obtained using a standard wet liposuction procedure under local anaesthesia, without the need for expansion in culture [50], [61].
ASCs are part of the stromal vascular fraction (SVF) of adipose tissue, together with a heterogeneous population of many other cell types, including preadipocytes, endothelial cells, pericytes, haematopoietic-lineage cells, and fibroblasts [62].
The regenerative features of the SVF are attributable to its paracrine effects: SVF cells secrete vascular endothelial growth factor, hepatocyte growth factor, and transforming growth factor-b in the presence of stimuli such as hypoxia and other growth factors and strongly influence the differentiation of stem cells, promoting angiogenesis and wound healing, and potentially aiding new tissue growth and development [50], [51], [52], [53], [54], [55], [56], [57].
In the mid-1990s, Coleman [31], [32], [33], [34], [35], [36], [37], [38] began to observe that the quality of the skin above the fat graft improved, not only as an effect of the filling but also with gradual improvement in the quality of the skin. There also appeared to be an improvement in the quality of the tissues into which fat is grafted, including softening of winkles, decreased size of pore and pigmentation improvement. He noted also that in the treatment of depressed scar, the fat grafted relieved the depression but also made the scar softer and sometimes it seemed to completely eliminate the scar, making it look like normal skin.
Rigotti et al. [63] reported that the transplantation of lipoaspirates containing adult ASCs is a highly effective therapeutic approach for the treatment of degenerative, chronic lesions induced as late effects of oncologic radiation treatments. Owing to the angiogenic factorsreleased from ASCs, lipofilling interrupted a vicious circle of vascular lesion, ischaemia, hyperpermeability, and fibrosis leading to increased ischaemia, and favoured the growth of a microvascular bed with the correct ratio of adipocytes to capillaries [63].
Because aspirated fat is relatively poor in adipose-derived stem cells, ASC condensation seems important for obtaining better regeneration and retention. Supplementation of stromal vascular fraction or ASCs can increase the ASC/adipocyte ratio in the graft. Clinical results of ASC supplementation remain controversial, but ASC condensation seems to lead to expanding applications of fat grafting into revitalization of stem cell-depleted tissue [64], [65].
4. Surgical technique
A standard fat grafting technique is commonly performed in three stages: harvesting of adipose tissue from a suitable donor site; processing of the lipoaspirate to eliminate cellular debris, acellular oil and excess of infiltrated solution, reinjection of the purified adipose tissue.
4.1. Harvesting
Many different techniques have been proposed for the removal of adipose tissue, all with the aim of minimizing adipocyte damage and increasing the survival of adipose tissue. Fat can be harvested by vacuum suction, syringe suction or surgical excision.
Negative pressure liposuction is faster than syringe aspiration and is an effective method for aspiration of large amounts of fat, but it causes massive destruction of adipocytes, greatly reducing the survival of fat graft [66].
High vacuum pressures of conventional liposuction may cause disruption of cellular structures in up to 90% of adipocytes [67].
Cannula size may also affect the viability of harvested fat [68]. The use of the excisional method and fat harvesting with large-bore cannulas reduce the occurrence of cellular ruptureand preserve the native tissue architecture.
Though optimal particle dimensions have yet to be determined, the consensus thus far is that the size must be large enough to preserve adipocytes and stromal cells and their anatomical relationship, but small enough to not limit diffusion of nutrients [69].
Larger harvesting cannulas facilitate the collection of larger fat particles and have been shown to facilitate better adipocyte viability and overall volume retention [70], [71].
This may be explained by the fact that larger diameter cannulas result in less shear stress, and more laminar flow of fat, leading to decreased tissue disruption during procurement [72].
Ozsoy et al. in their prospective study demonstrated a greater number of viable adipocytes with a 4-mm-diameter cannula compared with 2- or 3-mm cannulas [68].
Erdim et al. reported increased graft viability in fat harvested by liposuction using a 6-mm cannula compared with grafts obtained by 4-mm and 2-mm cannulas [73].
Campbell et al. found an inverse relationship between cellular damage and the diameter of the instrument used to extract fat [74].
Coleman [38] described a technique for fat harvest that minimized trauma to the adipocytes.
He used 3-mm incisions, a 3-mm, blunt edge, two-hole harvesting cannula connected to a 10-ml Luer-Lok syringe. The cannula is pushed through the harvest site (Fig. 2), as the surgeon uses digital manipulation to pull back on the plunger of the syringe and create a gentle negative pressure. A combination of slight negative pressure and the curetting action of the cannula through the tissues allows parcels of fat to move through the cannula and Luer-Lok aperture into the barrel of the syringe. When filled, the syringe is disconnected from the cannula and replaced with a plug that seals the Luer-Lok end of the syringe [38].
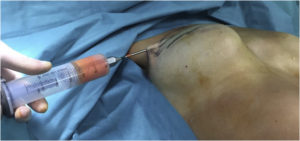
Fig. 1. Tissue infiltration with Klein’s solution.
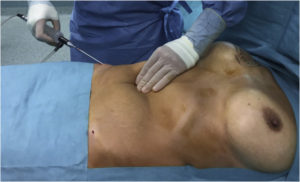
Fig. 1. Tissue infiltration with Klein’s solution.
Infiltration of the donor area has been another subject for debate in the literature. Fournier was the first who introduced the “dry technique”, in which no fluid was injected before the liposuction procedure, performed under general anesthesia or regional blocks [75]. He considered this technique more rapid and precise with a less tissue distortion, but the major disadvantages was the requirement of volume resuscitation in large-volume liposuction.
In 1993, Klein proposed a new method called tumescent technique in which a fluid solution (Klein’s solution) was injected into the donor site (Fig. 1). This improved the safety of large-volume liposuction because it eliminated the need for general anesthesia and reduced surgical haemorrhage [76], [77], [78].
Lidocaine alone has been associated with decreased adipocyte function, with Moore et al. finding transient changes to lipolysis and glucose transport in the presence of local anaesthetic. Interestingly, removal of lidocaine through washing harvested lipoaspirate returned these levels to normal [79].
Performing histomorphometric analysis and assessing cell viability of harvested adipocyte samples, Agostini et al. found no differences between the dry and the wet technique [80].
4.2. Processing
The goal of postharvest fat processing is to eliminate contaminants, including cellular debris, free oil, and other nonviable components of the lipoaspirate such as haematogenous cells [81]. These elements cause inflammation at the recipient site, which can be detrimental for the fat graft. Blood must be extracted because blood accelerates the degradation of the transplanted fat. Moreover, the injection of debris gives an erroneous impression of the volume of correction because the debris will be absorbed after a few hours [53] (Fig. 3).
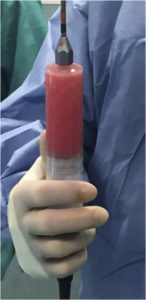
Fig. 3. Harvested fat before processing.
In addition, many authors believe that maximizing the number of adipose-derived mesenchymal stem cells in the grafted material improves graft viability [82]. Despite its widespread use, the methods and techniques used for grafting autologous adipose tissue remain varied, and the literature has thus far failed to reveal a single superior method for processing harvested fat.
Sedimentation is a little traumatic method with which it is possible to obtain a large number of vital and intact adipocytes [83].
However, this method contain smaller concentrations of stem cells and a significant amount of contaminating blood cells, aqueous and lipid with a proinflammatory effect and thus harmful to graft survival [82].
Recent publications further confirm this, demonstrating lower rates of decanted graft viability relative to centrifuged and washed specimens [83].
Filtration methods appear to eliminate contaminants, and maintain viable adipocytes and a large portion of adipose-derived mesenchymal stem cells. This processing technique may be more efficient in producing viable graft material for large-volume fat transfers [82]. A new technology developed is the Puregraft filtration system: it is a proprietary closed-membrane filtration system that was originally designed to prepare fat for isolation of the stromal vascular fraction. The technology it employs has yet to be publicly disclosed, although its mechanism is known to work by principles similar to a dialysis unit. Gerth et al. suggest that processing with the closed-membrane filtration system is less traumatic than centrifugation and that centrifugation is less able to clear free lipid, WBC, and RBC content from the fat graft. However, some authors have examined other filtration techniques and found no significant differences among the methods [84].Lipoaspirate filtered with cotton gauze, which results in concentrating the fat and separating it from the tumescent fluid, oily substances and cellular debris. The above method, as compared to centrifugation, showed no significant differences in vitality of transplant [85].
Washing the lipoaspirate has the goal of removing superfluous tumescent fluid, free lipids, and debris. This technique has previously been demonstrated to preserve both a large number of mesenchymal stem cells and a large number of adipocytes, thus satisfying both theories for graft survival [83].
Centrifugation is perhaps the most widely used technique for postharvest fat processing, and has previously been considered the criterion standard.
Coleman suggested a processing method that has gained popularity and has been since integrated in many fat-transfer clinical protocols [31], [67], [86]. The recommended centrifugation speed is 3000 rpm for 3 min.
Centrifugation separates the denser components from the less dense components to create layers. The upper level is the least dense and consists primarily of oil. The middle portion is primarily fatty tissue. The lowest layer is blood, water and any aqueous element [38].
This method obtains the highest possible concentration of stem cells within aspirate. It has also the increased content of angiogenic growth factors such as fibroblast growth factor(FGF) and vascular endothelial growth factor (VEGF) [87].
It separates adipocytes from substances that may degrade them, such as blood cells, lipids, proteases, and lipases but does not enhance immediate fat tissue viability. Centrifugation may enhance the total amount of transplanted fat, although excessive centrifugal force may damage intact adipocytes [88].
More aggressive processing with centrifugation has been used to dispose of fat cell debris, free triglycerides, ruptured membranes, and fragile fat cells. Ferraro et al. demonstrated that centrifugation with a force greater than 50 g resulted in damage to the structural integrity of adipose tissue, increased necrosis and apoptosis of cells, and decreased adipogenic differentiation capacity and tubule formation and he reported, also, better graft viability with low centrifugal forces [89]. However, more recently, Ferraro et al. reported an optimized centrifugal force at 250 g for 5 min, resulted in better density of adipose tissue, with good cell viability and increased ability to preserve significant number of progenitor cells [88].
Recent literature has demonstrated lower rates of graft viability after centrifugation relative to washing [83], although equivalent or superior results have been shown by some after “soft” centrifugation (400 g for 1 min) [90]. Kurita et al. recommended 1200 g for 3 min, as an optimized centrifugal force for obtaining good short- and long-term results in adipose transplantation [88]. Nevertheless, other research continues to support the equal effectiveness of standard centrifugation in preserving adipose-derived mesenchymal stem cells and producing viable in vivo grafts [91].
4.3. Implantation
Despite a long history of clinical use and evolution of techniques for fat transfer, no consensus exists to date on the best technique and the longevity of results; yet the principles of fat reimplantation are based on optimal recipient site vascularity for increased fat survival [92].
Graft through nutrition by tissue fluid absorption can survive up to 48 h. In the meantime, neovascularization progresses with the rate of about 1 mm per day. Therefore, the diameter of the deposit should not ideally be greater than 2 mm to avoid central necrosis [93], [94].
Through a skin incision of a size corresponding to the diameter of the cannula, the fat graft is inserted at the level of the anatomical region affected (Fig. 4). Small-gauge cannulas are thought to reduce trauma to the recipient site, thus reducing the risks of bleeding, haematoma formation, and poor graft oxygen diffusion [53].\
Fig. 4. Fat injection under depressed scar, resulting from implant breast reconstruction.
The placement cannulas are of a much smaller gauge, with only one hole at the distal end. Like the harvesting cannula, the proximal end of the infiltration cannula has a hub that will fit into a Luer-Lok syringe [38].
For various situations in the face and body, cannulas with different tip shapes, diameters, lengths, and curves can be used. The use of blunt cannulas allows placement of the fat parcels in a more stable, less traumatic manner. Through 2-mm incisions, the infiltration cannula is inserted and advanced through the recipient tissues into the appropriate plane [38]. Usually, through multiple access sites, multiple tunnels are created on insertion, but fat is injected only during withdrawal of the cannula [92]. As the cannula is withdrawn, the deposited fatty tissue parcels fall into the natural tissue planes as the host tissues collapse around them [38], [92].
Fat grafts are distributed in small aliquots and fanned out to varying depths in the soft tissue to avoid excessive interstitial pressure at the recipient site and overcrowding of the transplanted adipocytes [53]. Studies on fat-graft maintenance have demonstrated that mobile areas of the face, such as the glabella and lips, are less amenable to correction than are less-mobile areas, such as the malar and lateral cheek areas [92].
5. Conclusions
Fat grafting is a reconstructive and cosmetic procedure for patients with volume loss or contour deformities caused by disease, trauma, congenital defects, tumor extirpation, or the natural aging process [31], [92]. Actually fat is the closest to the ideal filler because it is readily available; easily obtainable, with low donor-site morbidity; repeatable; inexpensive; versatile; and biocompatible. Therefore, it is the standard against which all other fillers are comparing [31].
There is an abundance of literature supporting the efficacy of fat grafting in both aesthetic and reconstructive cases. Recent studies have shown the utility of adipose-derived stem cells in the improvement of wound healing, describing their ability to regenerate soft tissues and their remodeling capacity provided by their unique cytokine and growth factor profiles [95].
Despite ongoing concerns about survival and longevity of fat grafts after implantation and unpredictability of long-term outcome, fat has been successfully used as filler in many different clinical situations.
In plastic surgery, lipofilling is widely used in breast augmentation and reconstruction [96], [97], [98], [99], [100], [101] and in volume and contour deformities of the trunk [102], [103], [104] and lower limbs [105], [106].
Adipose-derived stem cells have also been shown to play a role in antiaging and skin regeneration by forming tissue consisting of hypodermis, dermis, and epidermis [91]. Also for this reason, fat grafts have an important role in the treatment of facial hemiatrophy and lipodystrophy [107], [108], [109], [110], in recontouring and rejuvenation of the aging face [34], [111], [112], [113], [114] and the hands [115], [116], [117], [118], [119], [120], in the treatment of depressed or altered scars [121], [122], [123], [124], [125], [126], [127], [128], [129] and also in the scleroderma treatment [129]. It is this regenerative capacity that is of particular interest also in chronic wound, including burns and ulcers wound therapy [130], [131], [132], [133], [134], [135].
It has also been successfully used in cleft lip and palate surgery, in orbital reconstructionafter tumor extirpation and in the treatment of painful extremity neuromas [92]. It has been useful in temporomandibular joint surgery, for treatment of ankylosis and prevention of fibrosis and heterotopic ossification around total joint prosthesis; in neurosurgery for spine and skull base surgeries to treat or prevent cerebrospinal fluid leaks as well as in otolaryngology for obliteration of ear, frontal sinus cavities and vocal cord augmentation [92].
The regenerative characteristics of adipose stem cells, the ease and the high availability of adipose tissue and the great diffusion of fat graft in the various medical disciplines have, in recent decades, been a major interest in new and more secure isolation techniques of stem cells [136], [137], [138], [139]. The extraction and implantation of stem cells has reversed, and is revealing, very high regenerative capacity for a large number of different organs and tissues, opening up new perspectives for regenerative medicine.
Ethical approval
N/A.
Sources of funding
N/A.
Author contribution
Edoardo Raposio: study design and data analysis.
Michele P. Grieco: management of clinical cases.
Elisa Bellini: data collection and writing the manuscript.
Conflicts of interest
None.
Unique Identifying Number (UIN)
N/A.
Guarantor
Prof. Edoardo Raposio is the Guarantor of the study.
References
- [1]
-
E. Billings Jr., J.W. May Jr.Historical review and present status of free fat graft auto transplantation in plastic and reconstructive surgeryPlast. Reconstr. Surg., 83 (1989), pp. 368-381
- [2]
-
Van der MeulenConsidérations générales sur les greffes graisseuses et séro-graisseuses épiplöiques et leurs principals applications(1919)[these medicine] Paris
- [3]
-
G.A. NeuberFett transplantationVerl Dtsch. Ges. Chir., 22 (1893), p. 66
- [4]
-
E. LexerFreie fett transplantationDtsch. Med. Wochenschr., 36 (1910), p. 640
- [5]
-
E. LexerZwanzig jahre transplantatiosforshung in der chirurgieArch. Klein Chir., 138 (1925), p. 294
- [6]
-
A. Mojallal, J.L. FoyatierHistorique de l’utilisation du tissu adipeux comme produit de comblement en chirurgie plastiqueAnn. Chir. Plast. Esthet., 49 (2004), pp. 419-425
- [7]
-
P. BrunningContribution à l’étude des greffes adipeusesBull. Mem. Acad. R. Med. Belg., 28 (1919), p. 440
- [8]
-
E. Hollander, M. JosephCosmetic surgery. Handbuch der KosmetikVeriag von Velt, Leipzig, Germany (1912), pp. 690-691
- [9]
-
C. MillerCannula Implants and Review of Implantation Techniques in Esthetic SurgeryThe Oak Press, Chicago (1926)
- [10]
-
M. CzernyReconstruction of the breast with a lipomaChir. Kongr. Verh., 2 (1895), p. 216
- [11]
-
N. Bertozzi, M. Pesce, P. Santi, E. RaposioTissue expansion for breast reconstruction: methods and techniquesAnn. Med. Surg., 21 (2017), pp. 34-44
- [12]
-
E. EitnerFettplastik bei GesichtsatrophieMed. Klin., 27 (1931), p. 624
- [13]
-
L.A. PeerLoss of weight and volume in human fat graft, with postulation of a « cell survival theoryPlast. Reconstr. Surg., 5 (1950), p. 217
- [14]
-
G. Boering, A.J. HuffstadtThe use of derma-fat grafts in the faceBr. J. Plast. Surg., 20 (1967), p. 172
- [15]
-
C.P. Sawhney, T.N. Banerjee, R.N. ChakravartiBehaviour of dermal fat transplantsBr. J. Plast. Surg., 22 (1969), p. 169
- [16]
-
H.O. BamesAugmentation mammaplasty by lipotransplantPlast. Reconstr. Surg., 11 (1953), p. 404
- [17]
-
F. ScrocherFettgewebsverpflanzung bei zu kleiner BrustMunchen Med. Wochenschr., 99 (1957), p. 489
- [18]
-
A. Fischer, G. FischerFirst surgical treatment for molding body’s cellulite with three 5 mm incisionsBull. Int. Acad. Cosmet. Surg., 3 (1976), p. 35
- [19]
-
Y. IllouzBody contouring by lipolysis: a 5 year experience with over 3000 casesPlast. Reconstr. Surg., 72 (1983), p. 511
- [20]
-
L.S. ToledoSyringe liposculpture for face and bodyAnnals of the International Symposium “Recent Advances in Plastic Surgery”, Estadao, Sao Paulo, Brazil(1989), p. 177
- [21]
-
L.S. ToledoSyringe liposculptureClin. Plast. Surg., 23 (1996), pp. 683-693
- [22]
-
P. FournierMicrolipoextraction et microlipoinjectionRev. Chir. Esthet., 10 (1985), p. 40
- [23]
-
P.F. FournierLiposculpture: the Syringe TechniqueArnette Blackwell, Paris (1991), p. 412
- [24]
-
P. FournierFacial recontouring with fat graftingDermatol. Clin., 8 (1989), p. 523
- [25]
-
M. BircollCosmetic breast augmentation utilizing autologous fat and liposuction techniquePlast. Reconstr. Surg., 79 (1987), pp. 267-271
- [26]
-
L. Hang-Fu, G. Marmolya, D.H. FeiglinLiposuction fat-fillant implant for breast augmentation and reconstructionAesthet. Plast. Surg., 19 (1995), p. 427
- [27]
-
P.B. Rosen, N.E. HugoAugmentation mammaplasty by cadaver fat allograftsPlast. Reconstr. Surg., 82 (1988), p. 525
- [28]
-
J. Haik, R. Talisman, J. Tamir, J. Frand, E. Gazit, J. Schibi, et al.Breast augmentation with fresh-frozen homologous fat graftsAesthet. Plast. Surg., 25 (2001), p. 292
- [29]
-
R. EllenbogenFree autogenous pearl fat grafts in the face-a preliminary report of a rediscovered techniqueAnn. Plast. Surg., 16 (1986), pp. 179-194
- [30]
-
L.A. PeerThe neglected free fat graftPlast. Reconstr. Surg., 1956 (18) (1946), pp. 233-250
- [31]
-
S.R. ColemanStructural fat grafts: the ideal filler?Clin. Plast. Surg., 28 (2001), p. 111
- [32]
-
S.R. ColemanHand rejuvenation with structural fat graftingPlast. Reconstr. Surg., 110 (2002), p. 1731discussion 1745
- [33]
-
S.R. ColemanLong-term survival of fat transplants: controlled demonstrationsAesthet. Plast. Surg., 19 (1995), p. 421
- [34]
-
S.R. ColemanFacial recontouring with lipostructureClin. Plast. Surg., 24 (1997), p. 347
- [35]
-
C. Tremolada, G. Palmieri, C. RicordiAdipocyte transplantation and stem cells: plastic surgery meets regenerative medicineCell Transplant., 19 (2010), pp. 1217-1223
- [36]
-
Report on autologous fat transplantation. ASPRS Ad-Hoc committee on new proceduresPlast. Surg. Nurs., 1987 (7) (September 30, 1987), pp. 140-141
- [37]
-
H. Neuhof, S. HirshfeldThe Transplantation of TissuesD. Appleton, New York (1923), pp. 1-297
- [38]
-
S.R. ColemanStructural fat grafting: more than a permanent fillerPlast. Reconstr. Surg., 118 (2006), pp. 108S-120S
- [39]
-
L.A. PeerCell survival theory versus replacement theoryPlast. Reconstr. Surg., 16 (1955), pp. 161-168
- [40]
-
E. Raposio, N. BertozziHow to isolate a ready-to-use adipose-derived stem cells pellet for clinical applicationEur. Rev. Med. Pharmacol. Sci., 21 (2017), pp. 4252-4260
- [41]
-
D. Von Heimburg, K. Hemmrich, S. Haydarlioglu, et al.Comparison of viable cell yield from excised versus aspirated adipose tissueCells Tissues Organs, 178 (2004), p. 87
- [42]
-
T.P. Wolter, D. Von Heimburg, I. Stoffels, et al.Cryopreservation of mature human adipocytes: in vitro measurement of viabilityAnn. Plast. Surg., 55 (2005), p. 408
- [43]
-
H. Eto, H. Kato, H. Suga, et al.The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytesPlast. Reconstr. Surg., 129 (2012), pp. 1081-1092
- [44]
-
H. Kato, K. Mineda, H. Eto, et al.Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 monthsPlast. Reconstr. Surg., 133 (2014), pp. 303e-313e
- [45]
-
B.J. Philips, T.L. Grahovac, J.E. Valentin, et al.Prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft modelPlast. Reconstr. Surg., 132 (2013), pp. 845-858
- [46]
-
R.M. Garza, R.C. Rennert, K.J. Paik, et al.Studies in fat grafting: Part IV. Adipose-derived stromal cell gene expression in cell-assisted lipotransferPlast. Reconstr. Surg., 135 (2015), pp. 1045-1055
- [47]
-
V. Cervelli, M.G. Scioli, P. Gentile, E. Doldo, E. Bonanno, L.G. Spagnoli, A. OrlandiPlatelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenanceStem Cells Transl. Med., 1 (2012), pp. 206-220
- [48]
-
F. Stillaert, M. Findlay, J. Palmer, et al.Host rather than graft origin of Matrigel-induced adipose tissue in the murine tissue-engineering chamberTissue Eng., 13 (2007), pp. 2291-2300
- [49]
-
F.B. Stillaert, P. Blondeel, M. Hamdi, et al.Adipose tissue induction in vivoAdv. Exp. Med. Biol., 585 (2006), pp. 403-412
- [50]
-
G. Caruana, N. Bertozzi, E. Boschi, et al.Role of adipose-derived stem cells in chronic cutaneous wound healingAnn. Ital. Chir., 86 (2015), pp. 1-4
- [51]
-
A.J. Salgado, R.L. Reis, N.J. Sousa, et al.Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicineCurr. Stem Cell Res. Ther., 5 (2010), pp. 103-110
- [52]
-
E. Raposio, S. Bonomini, F. CalderazziIsolation of autologous adipose tissue-derived mesenchymal stem cells for bone repairOrthop. Traumatol. Surg. Res., 102 (2016), pp. 909-912
- [53]
-
F. Simonacci, N. Bertozzi, M.P. Grieco, E. Grignaffini, E. RaposioProcedure, applications, and outcomes of autologous fat graftingAnn. Med. Surg., 20 (2017), pp. 49-60
- [54]
-
C. Scanarotti, A.M. Bassi, M. Catalano, C. Guida, R. Coradeghini, C. Falugi, M. Aluigi, P. Santi, E.RaposioNeurogenic-committed human pre-adipocytes express CYP1A isoformsChem. Biol. Interact., 184 (2010), pp. 474-483
- [55]
-
E. Raposio, C. Guida, I. Baldelli, F. Benvenuto, M. Curto, L. Paleari, F. Filippi, R. Fiocca, G. Robello, P.L.SantiCharacterization and induction of human pre-adipocytesToxicol. In Vitro, 21 (2007), pp. 330-334
- [56]
-
R. Coradeghini, C. Guida, C. Scanarotti, R. Sanguineti, A.M. Bassi, A. Parodi, P.L. Santi, E. RaposioA comparative study of proliferation and hepatic differentiation of human adipose-derived stem cellsCells Tissues Organs, 191 (2010), pp. 466-477
- [57]
-
M.G. Aluigi, R. Coradeghini, C. Guida, C. Scanarotti, A.M. Bassi, C. Falugi, P. Santi, E. RaposioPre-adipocytes commitment to neurogenesis 1: preliminary localisation of cholinergic moleculesCell Biol. Int., 33 (2009), pp. 594-601
- [58]
-
E. Raposio, C. Guida, R. Coradeghini, C. Scanarotti, A. Parodi, I. Baldelli, R. Fiocca, P.L. SantiIn vitro polydeoxyribonucleotide effects on human pre-adipocytesCell Prolif., 41 (2008), pp. 739-754
- [59]
-
F. Simonacci, N. Bertozzi, E. Raposio, et al.Off-label use of adipose-derived stem cellsAnn. Med. Surg. (Lond), 25 (2017), pp. 44-51
- [60]
-
J. Justesen, S.B. Pedersen, K. Stenderup, et al.Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivoTissue Eng., 10 (2004), pp. 381-391
- [61]
-
R.H. Lee, B. Kim, I. Choi, H. Kim, H.S. Choi, K. Suh, Y.C. Bae, J.S. JungCharacterization and expression analysis of mesenchymal stem cells from human bone marrow are proved to be safe and reliable, allowing surgeons and adipose tissueCell Physiol. Biochem., 14 (2004), pp. 311-324
- [62]
-
F. Simonacci, N. Bertozzi, M.P. Grieco, E. Grignaffini, E. RaposioAutologous fat transplantation for breast reconstruction: a literature reviewAnn. Med. Surg. (Lond.), 12 (2016), pp. 94-100
- [63]
-
G. Rigotti, A. Marchi, M. Gali_e, et al.Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adiposederived adult stem cellsPlast. Reconstr. Surg., 119 (2007), pp. 1409-1422
- [64]
-
S. Kuno, K. YoshimuraCondensation of tissue and stem cells for fat graftingClin. Plast. Surg., 42 (2015), pp. 191-197
- [65]
-
S.F. Kølle, A. Fischer-Nielsen, A.B. Mathiasen, J.J. Elberg, R.S. Oliveri, P.V. Glovinski, J. Kastrup, M.Kirchhoff, B.S. Rasmussen, M.L. Talman, C. Thomsen, E. Dickmeiss, K.T. DrzewieckiEnrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trialLancet, 382 (2013), pp. 1113-1120
- [66]
-
C.M. LewisComparison of the syringe and pump aspiration methods of lipoplastyAesthet. Plast. Surg., 15 (1991), pp. 203-208
- [67]
-
L.L. Pu, S.R. Coleman, X. Cui, et al.Autologous fat grafts harvested and refined by the Coleman technique: a comparative studyPlast. Reconstr. Surg., 122 (2008), pp. 932-937
- [68]
-
Z. Ozsoy, Z. Kul, A. BilirThe role of cannula diameter in improved adipocyte viability: a quantitative analysisAesthet. Surg. J., 26 (2006), pp. 287-289
- [69]
-
T.M. Gause II, R.E. Kling, W.N. Sivak, K.G. Marra, J.P. Rubin, L.E. KokaiParticle size in fat graft retention: a review on the impact of harvesting technique in lipofilling surgical outcomesAdipocyte, 3 (2014), pp. 273-279
- [70]
-
J.C. Kirkham, J.H. Lee, M.A. Medina III, M.C. McCormack, M.A. Randolph, W.G. Austen Jr.The impact of liposuction cannula size on adipocyte viabilityAnn. Plast. Surg., 69 (2012), pp. 479-481
- [71]
-
D. Tambasco, V. Arena, V. Finocchi, F. Grussu, D. CervelliThe impact of liposuction cannula size on adipocyte viabilityAnn. Plast. Surg., 73 (2014), pp. 249-251
- [72]
-
J.C. Kirkham, J.H. Lee, W.G. AustenFat graft survival: physics matters: invited commentary to “The impact of liposuction cannula size on adipocyte viability”Ann. Plast. Surg., 73 (2014), p. 359
- [73]
-
M. Erdim, E. Tezel, A. Numanoglu, et al.The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental studyJ. Plast. Reconstr. Aesthet. Surg., 62 (2009), pp. 1210-1214
- [74]
-
GL Campbell, N. Laudenslager, J. NewmanThe effect of mechanical stress on adipocyte morphology and metabolismAm. J. Cosmet. Surg., 4 (1987), pp. 89-94
- [75]
-
P.F. Fournier, F.M. OtteniLipodissection in body sculpturing: the dry procedurePlast. Reconstr. Surg., 72 (1983), pp. 598-609
- [76]
-
J.A. KleinThe tumescent technique for liposuction surgeryAm. J. Cosmet. Surg., 4 (1987), pp. 263-267
- [77]
-
J.A. KleinTumescent technique for local anesthesia improves safety in large volume liposuctionPlast. Reconstr. Surg., 92 (1993), pp. 1085-1098
- [78]
-
J.A. KleinTumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuctionJ. Dermatol. Surg. Oncol., 16 (1990), pp. 248-263
- [79]
-
J.H. Moore Jr., J.W. Kolaczynski, L.M. Morales, et al.Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaineAesthet. Plast. Surg., 19 (1995), pp. 335-339
- [80]
-
T. Agostini, D. Lazzeri, A. Pini, et al.Wet and dry techniques for structural fat graft harvesting: histomorphometric and cell viability assessments of lipoaspirated samplesPlast. Reconstr. Surg., 130 (2012), pp. 331e-339e
- [81]
-
K.A. GutowskiCurrent applications and safety of autologous fat grafts: a report of the ASPS fat graft task forcePlast. Reconstr. Surg., 124 (2009), pp. 272-280
- [82]
-
E.C. Cleveland, N.J. Albano, A. HazenRoll, spin, wash, or filter? Processing of lipoaspirate for autologous fat grafting: an updated, evidence-based review of the literaturePlastic Reconstr. Surg., 136 (2015), pp. 706-713
- [83]
-
A. Condé-Green, N.F. Gontijo De Amorim, I. PitanguyInfluence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative studyJ. Plast. Reconstr. Aesthet. Surg., 63 (2010), pp. 1375-1381
- [84]
-
D.J. Gerth, B. King, L. Rabach, R.A. Glasgold, M.J. GlasgoldLong-term volumetric retention of autologous fat grafting processed with closed-membrane filtrationAesthet. Surg. J., 34 (2014), pp. 985-994
- [85]
-
Y. Ramon, O. Shoshani, I.J. Peled, et al.Enhancing the take of injected adipose tissue by a simple method for concentrating fat cellsPlast. Reconstr. Surg., 115 (2005), pp. 197-201
- [86]
-
M.R. Kaufman, J.P. Bradley, B. Dickinson, et al.Autologous fat transfer consensus survey: trends in techniques for harvest, preparation, and application, and perception of short- and long-term resultsPlast. Reconstr. Surg., 119 (2007), pp. 323-331
- [87]
-
N. Pallua, A.K. Pulsfort, C. Suschek, T.P. WolterContent of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubationPlast. Reconstr. Surg., 123 (2009), pp. 826-833
- [88]
-
M. Kurita, D. Matsumoto, T. Shigeura, K. Sato, K. Gonda, K. Harii, K. YoshimuraInfluences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolationPlast. Reconstr. Surg., 121 (2008), pp. 1033-1041
- [89]
-
G.A. Ferraro, F. De Francesco, V. Tirino, C. Cataldo, F. Rossano, G. Nicoletti, F. D’AndreaEffects of a new centrifugation method on adipose cell viability for autologous fat graftingAesthet. Plast. Surg., 35 (2011), pp. 341-348
- [90]
-
L. Hoareau, K. Bencharif, A.C. Girard, et al.Effect of centrifugation and washing on adipose graft viability: a new method to improve graft efficiencyJ. Plast. Reconstr. Aesthet. Surg., 66 (2013), pp. 712-719
- [91]
-
H.M. Salinas, G.F. Broelsch, J.R. Fernandes, et al.Comparative analysis of processing methods in fat graftingPlast. Reconstr. Surg., 134 (2014), pp. 675-683
- [92]
-
D. Kakagia, N. PalluaAutologous fat grafting: in search of the optimal techniqueSurg. Innov., 21 (2014), pp. 327-336
- [93]
-
R.K. Khouri, G. Rigotti, E. Cardoso, et al.Megavolume autologous fat transfer: part I. Theory and principlesPlast. Reconstr. Surg., 133 (2014), pp. 550-557
- [94]
-
R.K. Khouri, G. Rigotti, E. Cardoso, et al.Megavolume autologous fat transfer: part II. Practice and techniquesPlast. Reconstr. Surg., 133 (2014), pp. 1369-1377
- [95]
-
A. Condé-Green, A.A. Marano, E.S. Lee, T. Reisler, L.A. Price, S.M. Milner, M.S. GranickFat grafting and adipose- derived regenerative cells in burn wound healing and scarring: a systematic review of the literaturePlast. Reconstr. Surg., 137 (2016), pp. 302-312
- [96]
-
E. Raposio, N. BertozziAutologous fat grafting and processing after breast reconstructionSurg. Chron., 22 (2017), pp. 66-72
- [97]
-
M. Gardani, N. Bertozzi, M.P. Grieco, M. Pesce, F. Simonacci, P.L. Santi, E. RaposioBreast reconstruction with anatomical implants: a review of indications and techniques based on current literatureAnn. Med. Surg., 21 (2017), pp. 96-104
- [98]
-
N. Bertozzi, M. Pesce, P. Santi, E. RaposioOne-stage immediate breast reconstruction: a concise reviewBiomed. Res. Int., 2017 (2017), p. 6486859
- [99]
-
N. Bertozzi, M. Pesce, P.L. Santi, E. RaposioOncoplastic breast surgery: comprehensive reviewEur. Rev. Med. Pharmacol. Sci., 21 (2017), pp. 2572-2585
- [100]
-
J. Fan, E. Raposio, J. Wang, R.E. NordströmDevelopment of the inframammary fold and ptosis in breast reconstruction with textured tissue expandersAesthet. Plast. Surg., 26 (2002), pp. 219-222
- [101]
-
I. Porro, A. Schenone, M. Fato, E. Raposio, E. Molinari, F. BeltrameAn integrated environment for plastic surgery support: building virtual patients, simulating interventions, and supporting intraoperative decisionsComput. Med. Imaging Graph, 29 (2005), pp. 385-394
- [102]
-
E. Delay, R. Sinna, K. Chekaroua, et al.Lipomodeling of Poland’s syndrome: a new treatment of the thoracic deformityAesthet. Plast. Surg., 34 (2010), pp. 218-225
- [103]
-
E. Grignaffini, M.P. Grieco, N. Bertozzi, M. Gandolfi, D. Palli, F.G. Cinieri, M. Gardani, E. RaposioPost-bariatric abdominoplasty: our experienceActa Biomed., 86 (2015), pp. 278-282
- [104]
-
M. Grieco, E. Grignaffini, F. Simonacci, E. RaposioAnalysis of complications in postbariatric abdominoplasty: our experiencePlast. Surg. Int., 2015 (2015), p. 209173
- [105]
-
A. Mojallal, M. Veber, C. Shipkov, et al.Analysis of a series of autologous fat tissue transfer for lower limb atrophiesAnn. Plast. Surg., 61 (2008), pp. 537-543
- [106]
-
M. Grieco, E. Grignaffini, F. Simonacci, D. Di Mascio, E. RaposioPost-bariatric body contouring: our experienceActa Biomed., 87 (2016), pp. 70-75
- [107]
-
A. Mori, G. Lo Russo, T. Agostini, J. Pattarino, F. Vichi, M. DiniTreatment of human immunodeficiency virus-associated facial lipoatrophy with lipofilling and submalar silicone implantsJ. Plast. Reconstr. Aesthet. Surg., 59 (2006), pp. 1209-1216
- [108]
-
A. Carr, K. Samaras, D.J. Chisholm, D.A. CooperPathogenesis of HIV-1- protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistanceLancet, 351 (1998), pp. 1881-1883
- [109]
-
K. Brinkman, J.A. Smeitink, J.A. RomijnReiss P.,Mitochondrial toxicity induced by nucleoside-analogue reverse- transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophyLancet, 354 (1999), pp. 1112-1115
- [110]
-
M. Grimaldi, P. Gentile, L. Labardi, E. Silvi, A. Trimarco, V. CervelliLipostructure technique in Romberg syndromeJ. Craniofac. Surg., 19 (2008), pp. 1089-1091
- [111]
-
A.K. Gosain, M.H. Klein, P.V. Sudhakar, R.W. ProstA volumetric analysis of soft-tissue changes in the aging midface using high-resolution MRI: implications for facial rejuvenationPlast. Reconstr. Surg., 115 (2005), pp. 1143-1152
- [112]
-
C. Le LouarnMidface region: functional anatomy, ageing process, indications and concentric malar liftAnn. Chir. Plast. Esthet., 54 (2009), pp. 411-420
- [113]
-
R.J. Rohrich, J.E. PessaThe fat compartments of the face: anatomy and clinical implications for cosmetic surgeryPlast. Reconstr. Surg., 119 (2007), pp. 219-227
- [114]
-
R.J. Rohrich, G.M. Arbique, C. Wong, S. Brown, J.E. PessaThe anatomy of suborbicularis fat: implications for periorbital rejuvenationPlast. Reconstr. Surg., 124 (2009), pp. 946-951
- [115]
-
D. Hoang, M.I. Orgel, v KulberHand rejuvenation: a comprehensive review of fat graftingJ. Hand Surg. Am., 41 (2016), pp. 639-644
- [116]
-
B. Teimourian, M. AdhamRejuvenation of the hand: fat injection combined with TCA peelAesthet. Surg. J., 20 (2000), pp. 70-71
- [117]
-
R.D. Bains, H. Thorpe, S. SouthernHand aging: patients’ opinionsPlast. Reconstr. Surg., 117 (2005), pp. 2212-2218
- [118]
-
R. Jakubietz, J.G. Grünert, D.F. Kloss, R. Meffert, K. Schmidt, M.G. JakubietzAging and aesthetic ideal of the handHautarzt, 60 (2009), pp. 220-225
- [119]
-
S.G. Fabi, M.P. GoldmanHand rejuvenation: our experienceDermatol. Surg., 38 (2012), pp. 1112-1127
- [120]
-
M. Streker, T. Reuther, N. Krueger, M. KerscherStabilized hyaluronic acid based gel of non-animal origin for skin rejuvenation: face, hand, and decolletageJ. Drugs Dermatol., 12 (2013), pp. 990-994
- [121]
-
R.K. Khouri, J.M. Smit, E. Cardoso, N. Pallua, L. Lantieri, I.M. Mathijssen, R.K. Khouri Jr., G. RigottiPercutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction?Plast. Reconstr. Surg., 132 (2013), pp. 280-1290
- [122]
-
M. Klinger, M. Marazzi, D. Vigo, M. TorreFat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reductionAesthet. Plast. Surg., 32 (2008), pp. 465-469
- [123]
-
F. Caviggioli, F. Villani, D. Forcellini, V. Vinci, F. KlingerScar treatment by lipostructure, UpdatePlast. Surg., 2 (2009), pp. 51-53
- [124]
-
M. Klinger, F. Caviggioli, F.M. Klinger, S. Giannasi, V. Bandi, B. Banzatti, D. Forcellini, L. Maione, B.Catania, V. VinciAutologous fat graft in scar treatmentJ. Craniofac. Surg., 24 (2013), pp. 1610-1615
- [125]
-
E. Raposio, N. Bertozzi, S. Bonomini, G. Bernuzzi, A. Formentini, E. Grignaffini, M. Pio GriecoAdipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapyWounds, 28 (2016), pp. 126-131
- [126]
-
E. Raposio, F. CalderazziFat grafting for chronic heel pain following surgery for adult flatfoot deformity: pilot studyFoot (Edinb), 31 (2017), pp. 56-60
- [127]
-
N. Bertozzi, F. Simonacci, M.P. Grieco, E. Grignaffini, E. RaposioThe biological and clinical basis for the use of adipose-derived stem cells in the field of wound healingAnn. Med. Surg., 20 (2017), pp. 41-48
- [128]
-
V. Cervelli, F. Nicoli, D. Spallone, S. Verardi, R. Sorge, M. Nicoli, A. BalzaniTreatment of traumatic scars using fat grafts mixed with platelet-rich plasma, and resurfacing of skin with the 1540 nm nonablative laserClin. Exp. Dermatol., 37 (2012), pp. 55-61
- [129]
-
G. Magalon, A. Daumas, N. Sautereau, J. Magalon, F. Sabatier, B. GranelRegenerative approach to scleroderma with fat graftingClin. Plast. Surg., 42 (2015), pp. 353-364viii-ix
- [130]
-
A. Mojallal, C. Lequeux, C. Shipkov, P. Breton, J.L. Foyatier, F. Braye, O. DamourImprovement of skin quality after fat grafting: clinical observation and an animal studyPlast. Reconstr. Surg., 124 (2009), pp. 765-774
- [131]
-
B.S. Atiyeh, M. CostagliolaCultured epithelial autograft (CEA) in burn treatment: three decades laterBurns, 33 (2007), pp. 405-413
- [132]
-
I. Jones, L. Currie, R. MartinA guide to biological skin substitutesBr. J. Plast. Surg., 55 (2002), pp. 185-193
- [133]
-
C.P. Zhang, X.B. FuTherapeutic potential of stem cells in skin repair and regenerationChin. J. Traumatol., 11 (2009), pp. 209-221
- [134]
-
J.J. Lataillade, C. Doucet, E. Bey, H. Carsin, C. Huet, I. Clairand, J.F. Bottollier-Depois, A. Chapel, I.Ernou, M. Gourven, L. Boutin, A. Hayden, C. Carcamo, E. Buglova, M. Joussemet, T. De Revel, P.GourmelonNew approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapyRegen. Med., 2 (2007), pp. 785-794
- [135]
-
A. Arno, A.H. Smith, P.H. Blit, M.A. Shehab, G.G. Gauglitz, M.G. JeschkeStem cell therapy: a new treatment for burns?Pharmaceuticals, 4 (2011), pp. 1355-1380
- [136]
-
E. Raposio, G. Caruana, M. Petrella, S. Bonomini, M.P. GriecoA standardized method of isolating adipose-derived stem cells for clinical applicationsAnn. Plast. Surg., 76 (2016), pp. 124-126
- [137]
-
E. Raposio, G. Caruana, S. Bonomini, G. LibondiA novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapyPlast. Reconstr. Surg., 133 (2014), pp. 1406-1409
- [138]
-
E. Raposio, N. BertozziIsolation of ready-to-use Adipose-derived Stem Cell (ASC) pellet for clinical applications and a comparative overview of alternate methods for ASC isolationCurr. Protoc. Stem Cell Biol., 16 (41) (2017)1F.17.1-1F.17
- [139]
-
E. Raposio, F. Simonacci, R.E. PerrottaAdipose-derived stem cells: comparison between two methods of isolation for clinical applicationsAnn. Med. Surg., 20 (2017), pp. 87-91